Chromatography
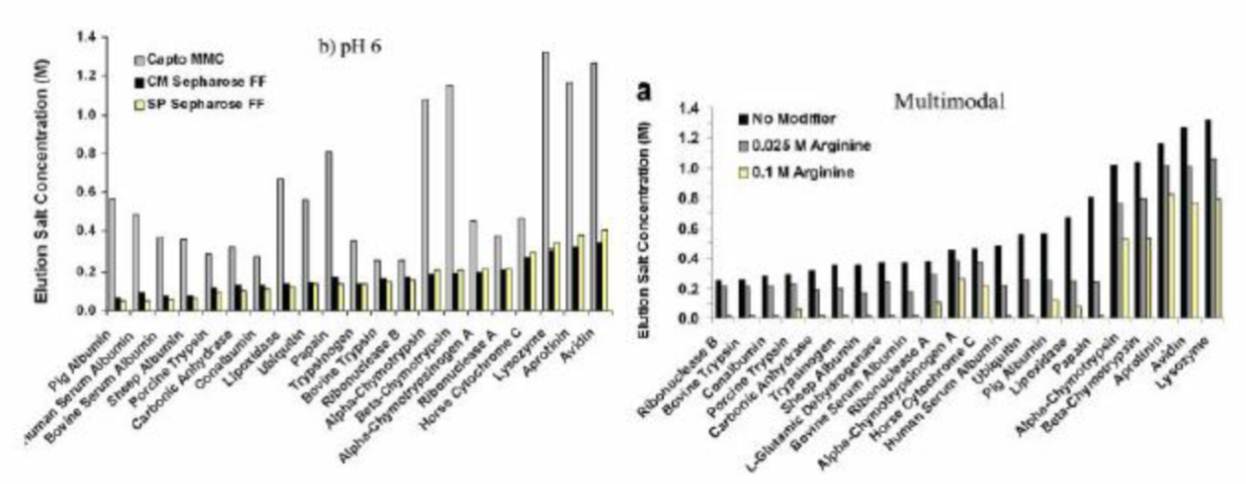
Batch and column chromatography experiments are used extensively in our laboratory to study protein-ligand interactions by generating adsorption isotherm parameters and protein retention models with a variety of chromatographic resins including ion exchange (IEX), hydrophobic interaction (HIC), hydroxyapatite, and multimodal (MM) resins. We employ both isocratic and gradient elution modes in our column chromatography studies in order to develop predictive models of protein retention behavior. Chromatographic studies have been carried out using diverse model protein libraries as well as homologous series. Current research efforts focus on understanding the interactions between antibodies and antibody-related proteins with multimodal ligands.
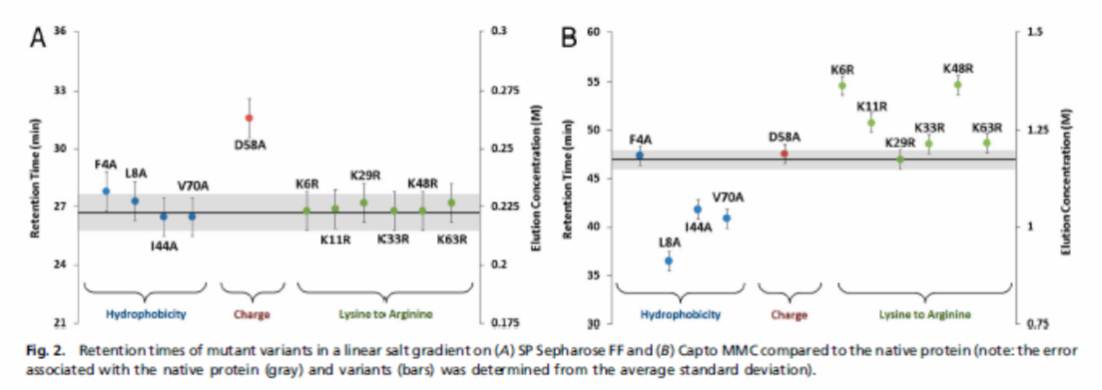
To study particular physiochemical properties (e.g. charge, hydrophobicity) in detail, libraries of homologous protein variants were developed. For ion-exchange studies, charge ladder libraries were created by succinylating or acetylating lysine residues of horse cytochrome C and lysozyme to create homologous proteins with different charge profiles. For our multimodal investigation, we have constructed homologous series of ubiquitin and cold-shock protein B (shown below), to probe the effects of point mutations in charged and hydrophobic areas on the protein’s retention in multimodal systems.
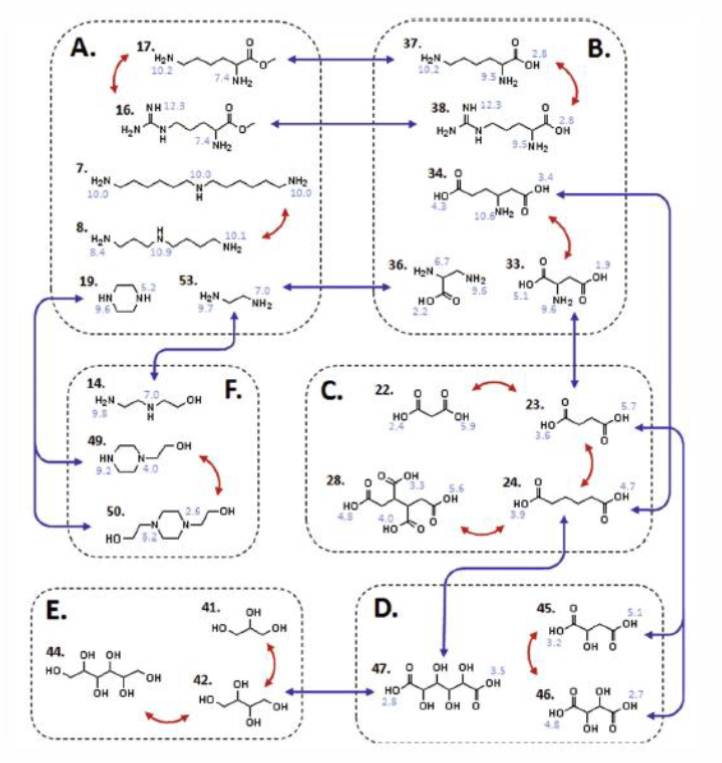
Additional experiments have been performed in the presence of various fluid phase modifiers including organic molecules, various salt types, folding agents, and denaturants in order to introduce new and improved selectivities between proteins in a given separation. Our group also employs high-throughput batch chromatography experiments to screen a larger range of fluid phase binding/elution conditions on a variety of resins to identify novel protein-ligand binding interactions and selectivity. One of our focuses is to investigate libraries of homologous multimodal ligands and understand how changes in geometry and physiochemical properties (e.g. charge, hydrophobicity, hydrogen bonding) affect the ligand’s selectivity for particular proteins.
Advancements in displacement chromatography are another focus of the group. Displacement chromatography can be used to both concentrate and purify molecules and has been used to achieve baseline resolution between previously inseparable molecules. Several small molecule displacer libraries have been developed and screened for selective protein separations in IEX, HIC and hydroxyapatite systems and we are currently identifying displacer systems for multimodal resins.
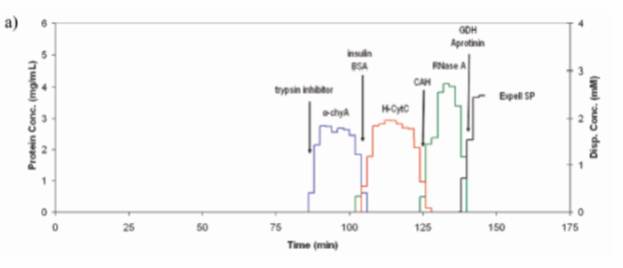
Displacer libraries are first screened in a high-throughput batch mode using a robotics system, with successful candidates then employed and developed further in columns to achieve highly-selective protein separations (as seen here).
Selected References:
- M.A. Holstein, et al. J. Chrom. A (2012). Improving selectivity in multimodal chromatography using controlled pH gradient elution.
- M.A. Holstein, et al. Biotechnol. Bioeng. (2011). Mobile Phase Modifier Effects in Multimodal Cation Exchange Chromatography.
- S.T. Evans, M.A. Holstein and S.M. Cramer. Anal. Chem. (2011). Detection of Trace Proteins in Multicomponent Mixtures Using Displacement Chromatography.
- C.J. Morrison, P. Gagnon and S.M. Cramer. J. Chrom. A (2010). Unique selectivity windows using selective displacers/eluents and mobile phase modifiers on hydroxyapatite.
- C.J. Morrison, J.A. Moore and S.M. Cramer. Chromatographia (2010). Alkyl Based Selective Displacers for Protein Purification in Ion Exchange Chromatography.
- Y. Hou, et al. J. Chrom. A (2010). Effects of urea induced protein conformational changes on ion exchange chromatographic behavior.
- W.K. Chung, et al. J. Chrom. A (2010). Investigation of protein binding affinity in multimodal chromatographic systems using a homologous protein library.
- W.K. Chung, et al. Langmuir (2010). Utilization of Lysozyme Charge Ladders to Examine the Effects of Protein Surface Charge Distribution on Binding Affinity in Ion Exchange Systems.
